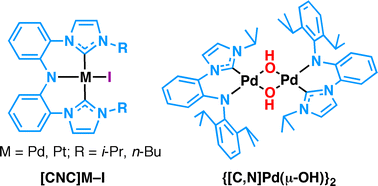
With a view to applications in bifunctional catalysis, a modular cross-coupling strategy has been used to prepare amine bis(imidazolium) salts (3a and 3b) and an amine mono(imidazolium) salt (6) as precursors to chelating amido-NHC ligands. Treating the pro-ligands 3 with 3 equivalents of the bulky base KHMDS and Pd(OAc)2 or PtCl2(COD) gave the four amido bis(N-heterocyclic carbene) pincer complexes [CNC-R]M-I [M = Pd (7) or Pt (8); R = i-Pr (a) or n-Bu (b)], including the first examples of platinum complexes of a CNC ligand. The reaction of 7a with AgOTf in pyridine gave the cationic complex {[CNC-i-Pr]Pd-py}OTf (9a). Heating a mixture of amine mono(imidazolium) salt 6 with PdCl2 or K2PtCl4, K2CO3 and KI in pyridine at 100 °C gave the complexes [C,NH]MI2py [M = Pd (10) or Pt (11)], in which the amine arm of the NHC ligand is not deprotonated and does not coordinate to the metal. For a solution of 10 in 1,4-dioxane, deprotonation of the amine occurred in a biphasic reaction with aqueous KOH at 40 °C, giving the dimeric amido complex {[C,N]Pd(μ-OH)}2 (12). The more inert Pt analogue 11 was unreactive under the same conditions. Solid-state structures of the complexes 7a, 7b, 9a, 10, 11 and 12 have been determined by single crystal X-ray diffraction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...