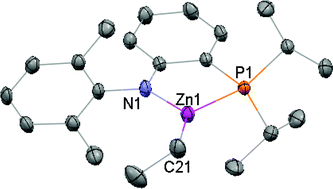
A series of diarylamido phosphine ligands of the type N-(2-dihydrocarbylphosphinophenyl)-2,6-dialkylanilide 1a–d have been prepared and employed to investigate the coordination chemistry of zinc. Protonolysis of ZnMe2 with one equivalent of N-(2-diphenylphosphinophenyl)-2,6-dimethylaniline (H[1a]) produced a mixture of [1a]ZnMe (2a) and Zn[1a]2 (4a), whereas that involving ZnEt2 gave exclusively the three-coordinate [1a]ZnEt (3a). In contrast, treatment of ZnR2 (R = Me, Et) with N-(2-diphenylphosphinophenyl)-2,6-diisopropylaniline (H[1b]), N-(2-diisopropylphosphinophenyl)-2,6-dimethylaniline (H[1c]), or N-(2-diisopropylphosphinophenyl)-2,6-diisopropylaniline (H[1d]) under similar conditions generated quantitatively the corresponding three-coordinate zinc methyl 2b–d and zinc ethyl 3b–d. The bis-ligand complexes 4a,b,d were isolated by either protonolysis of alkyls 2–3 with one equivalent of H[1] or metathesis of ZnX2 (X = Cl, OAc) with the corresponding lithium derivatives 5. Attempts to prepare [1a–d]ZnX (X = Cl, OAc) were not successful regardless of stoichiometry of the starting materials employed. Alcoholysis of zinc alkyls 2–3 led undesirably to protonation on the amido nitrogen donor of 1, highlighting perhaps its higher basicity than alkyls. The reaction of ZnCl2 with H[1c] generated the phosphorus-bound adduct {H[1c]ZnCl(μ-Cl)}2 (6c). Interestingly, attempts to deprotonate 6c with n-BuLi produced unexpectedly the alkylated product [1c]Zn(n-Bu) (7c) instead of [1c]ZnCl; analogous reactions employing NEt3 led to Lewis base substitution to give H[1c] and [ZnCl2(NEt3)]2. Structural characterization of all new compounds was achieved by multi-nuclear NMR spectroscopy (1H, 13C, 31P, and 7Li) and X-ray crystallography (2c–d, 3c, 4d, 5c–d, and 6c) where appropriate. On the basis of the NMR and X-ray data, in combination with the synthetic investigations, the steric nature of these amido phosphine ligands is recognized to follow the order of 1a < 1b < 1c < 1d. Interestingly, zinc alkyls 2–3 are all active initiators for catalytic ring-opening polymerization of ε-caprolactone whereas the bis-ligand complexes 4 are not.

 Please wait while we load your content...
Please wait while we load your content...