The potential energy surfaces of the reactions of Cl4MCH2 (M = Cr, Mo, W, Ru, Re) with ethylene, models of potential chain-carrying catalysts and olefins respectively in the transition metal-catalyzed olefin metathesis reaction, have been explored using density functional theory at the B3LYP/LACVP* level of theory. In Cl4MCH2 (M = Cr, Ru), the carbenoid complexes Cl3MCH2–Cl have been found to be more stable than the corresponding carbene Cl4M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 complexes whereas in Cl4MCH2 (M = Mo, W, Re) the carbene complexes are more stable than the carbenoid complexes. The carbenoid complexes have been found not to favor the formation of metallacyclobutanes, a key step in the olefin metathesis reaction according to the Herrison–Chauvin mechanism, indicating that the active species for the metathesis reaction is a carbene complex and not a carbenoid complex. Therefore, even though the formation of metallacyclobutanes through formal [2+2] cycloaddition has been found to be a low-barrier process with each of the metal carbene complexes investigated, metathesis is likely to occur only in Cl4MCH2(M = Mo, W, Re) but not in Cl4MCH2(M = Cr, Ru) since the reaction surface in the latter complexes is likely to be populated by the carbenoid complex rather than the carbene complex. In Cl4W
CH2 complexes whereas in Cl4MCH2 (M = Mo, W, Re) the carbene complexes are more stable than the carbenoid complexes. The carbenoid complexes have been found not to favor the formation of metallacyclobutanes, a key step in the olefin metathesis reaction according to the Herrison–Chauvin mechanism, indicating that the active species for the metathesis reaction is a carbene complex and not a carbenoid complex. Therefore, even though the formation of metallacyclobutanes through formal [2+2] cycloaddition has been found to be a low-barrier process with each of the metal carbene complexes investigated, metathesis is likely to occur only in Cl4MCH2(M = Mo, W, Re) but not in Cl4MCH2(M = Cr, Ru) since the reaction surface in the latter complexes is likely to be populated by the carbenoid complex rather than the carbene complex. In Cl4W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 the kinetic and thermodynamic preference of the productive [2+2] pathway leading to the metallacyclobutane over the [3+2] pathway is unambiguous whereas in Cl4MoCH2 the [3+2] pathway is likely to be competitive with the [2+2] pathway. The metallacyclobutane formation in W and Re has been found to have lower barriers than in Mo, suggesting that the W and Re complexes may have a greater metathesis activity than the Mo complex.
CH2 the kinetic and thermodynamic preference of the productive [2+2] pathway leading to the metallacyclobutane over the [3+2] pathway is unambiguous whereas in Cl4MoCH2 the [3+2] pathway is likely to be competitive with the [2+2] pathway. The metallacyclobutane formation in W and Re has been found to have lower barriers than in Mo, suggesting that the W and Re complexes may have a greater metathesis activity than the Mo complex.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 complexes whereas in Cl4MCH2 (M =
CH2 complexes whereas in Cl4MCH2 (M = ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 the kinetic and thermodynamic preference of the productive [2+2] pathway leading to the
CH2 the kinetic and thermodynamic preference of the productive [2+2] pathway leading to the 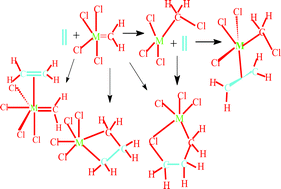

 Please wait while we load your content...
Please wait while we load your content...