Chemical reactions of Pt(pap)Cl2 [pap = 2-(phenylazo)pyridine] with the N,S-donor atom ligands, 2-alkylthioanilines (HL, (H2N⁁SR), where R = Me, -CH2Ph, -CH2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) in acetonitrile solvent under alkaline conditions yielded mixed chelate donor–acceptor complexes, [Pt(pap)(HN⁁SR)]+ (1+, R = Me), [Pt(pap)(HN⁁S)] (2, HN⁁S = 2-amidothiophenolate, R = Me, -CH2Ph, -CH2-CH
CH2) in acetonitrile solvent under alkaline conditions yielded mixed chelate donor–acceptor complexes, [Pt(pap)(HN⁁SR)]+ (1+, R = Me), [Pt(pap)(HN⁁S)] (2, HN⁁S = 2-amidothiophenolate, R = Me, -CH2Ph, -CH2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) and [Pt(pap)(RN⁁S)] (RN⁁S = 2-(N-alkyl)amidothiophenolate 3 and 4, R = -CH2Ph, -CH2-CH
CH2) and [Pt(pap)(RN⁁S)] (RN⁁S = 2-(N-alkyl)amidothiophenolate 3 and 4, R = -CH2Ph, -CH2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). Unusual types of 1,4-alkyl group migration associated with simultaneous S–C bond cleavage and N–C bond formation were observed for R = -CH2Ph, -CH2-CH
CH2). Unusual types of 1,4-alkyl group migration associated with simultaneous S–C bond cleavage and N–C bond formation were observed for R = -CH2Ph, -CH2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. However, for R = Me, only S–C bond cleavage occurred producing compound 2 at a higher temperature. Under identical experimental conditions the reaction of Pt(bpy)Cl2 [bpy = 2,2′-bipyridine] with 2-methylthioaniline afforded [Pt(bpy)(HN⁁SR)]+ (5+, R = Me) as the only product with no S–C bond activation. The complexes have been characterized by 1H NMR, UV-vis-NIR, ESI-MS, EPR and cyclic voltammetry studies. Single-crystal X-ray structures of the complexes, [1][OTf] (OTf = trifluoromethanesulfonate), 2, 3, 4 and [5][OTf] are reported. The Pt-pap complexes (1+ and 2–4) showed intense interligand charge transfer (ILCT) transition in the NIR-region (>800 nm). This band in the Pt-bpy analogue, [5]+ shifted to a much higher energy, at 545 nm. The cationic complex, [1][OTf] displayed two reversible responses at −0.21 and −0.94 V along with two irreversible anodic responses at 0.48 and 0.90 V. The molecular compounds, 2, 3 and 4 showed two reversible waves near −0.60 and −1.30 V and an irreversible anodic response near 0.5 V. The response at the anodic potential is presumably due to oxidation of 2-amidothioether/2-amidothiophenolate ligand while the reversible responses at cathodic potentials are due to successive reductions of the azo chromophore of the coordinated pap ligand. The redox processes are characterized by EPR and spectroelectrochemistry. Density-functional theory calculations were employed to confirm the structural features and to support the spectral and redox properties of the complexes.
CH2. However, for R = Me, only S–C bond cleavage occurred producing compound 2 at a higher temperature. Under identical experimental conditions the reaction of Pt(bpy)Cl2 [bpy = 2,2′-bipyridine] with 2-methylthioaniline afforded [Pt(bpy)(HN⁁SR)]+ (5+, R = Me) as the only product with no S–C bond activation. The complexes have been characterized by 1H NMR, UV-vis-NIR, ESI-MS, EPR and cyclic voltammetry studies. Single-crystal X-ray structures of the complexes, [1][OTf] (OTf = trifluoromethanesulfonate), 2, 3, 4 and [5][OTf] are reported. The Pt-pap complexes (1+ and 2–4) showed intense interligand charge transfer (ILCT) transition in the NIR-region (>800 nm). This band in the Pt-bpy analogue, [5]+ shifted to a much higher energy, at 545 nm. The cationic complex, [1][OTf] displayed two reversible responses at −0.21 and −0.94 V along with two irreversible anodic responses at 0.48 and 0.90 V. The molecular compounds, 2, 3 and 4 showed two reversible waves near −0.60 and −1.30 V and an irreversible anodic response near 0.5 V. The response at the anodic potential is presumably due to oxidation of 2-amidothioether/2-amidothiophenolate ligand while the reversible responses at cathodic potentials are due to successive reductions of the azo chromophore of the coordinated pap ligand. The redox processes are characterized by EPR and spectroelectrochemistry. Density-functional theory calculations were employed to confirm the structural features and to support the spectral and redox properties of the complexes.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) in
CH2) in ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) and
CH2) and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). Unusual types of 1,4-alkyl
CH2). Unusual types of 1,4-alkyl ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. However, for R = Me, only S–C bond cleavage occurred producing compound 2 at a higher temperature. Under identical experimental conditions the reaction of
CH2. However, for R = Me, only S–C bond cleavage occurred producing compound 2 at a higher temperature. Under identical experimental conditions the reaction of 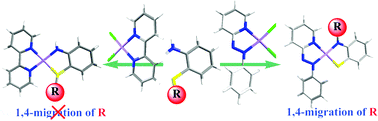

 Please wait while we load your content...
Please wait while we load your content...