The 2-cyano-2-isonitrosoacetamide, NC-C(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OH)-C(O)NH2 (1), its Na+, Cs+ salts and four silver(I) complexes with N-donor ligands were synthesized and characterized using a variety of techniques including IR, UV-vis spectroscopy, solid state photoluminescence, X-ray analysis, and solution electrical conductivity. All four reported here Ag(I) complexes were crystallographically characterized and revealed completely different structures. Thus, the combination of chelate and bridging function of the cyanoxime anion in Ag{NC-C(N
OH)-C(O)NH2 (1), its Na+, Cs+ salts and four silver(I) complexes with N-donor ligands were synthesized and characterized using a variety of techniques including IR, UV-vis spectroscopy, solid state photoluminescence, X-ray analysis, and solution electrical conductivity. All four reported here Ag(I) complexes were crystallographically characterized and revealed completely different structures. Thus, the combination of chelate and bridging function of the cyanoxime anion in Ag{NC-C(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2} (complex 6) leads to a unique layered 2D coordination polymer of silver(I) with pronounced argentophilic interactions at 3.194 Å. The structure of Ag{NC-C(N
O)-C(O)NH2} (complex 6) leads to a unique layered 2D coordination polymer of silver(I) with pronounced argentophilic interactions at 3.194 Å. The structure of Ag{NC-C(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2}·2Pic (Pic = 2-methylpyridine; complex 7) represents the monomeric complex containing bidentate chelate anion 1−. The crystal structure of monomeric Ag{NC-C(N
O)-C(O)NH2}·2Pic (Pic = 2-methylpyridine; complex 7) represents the monomeric complex containing bidentate chelate anion 1−. The crystal structure of monomeric Ag{NC-C(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2}·2NH3 (complex 8) reveals the formation of a linear Ag(NH3)2+ cation non bonded to the metal cyanoxime anion 1−. This is the first structure of a silver(I) diammine cation with an oxime-based anion. Similarly to 8, the crystal structure of Ag(14ane[N4]){NC-C(N
O)-C(O)NH2}·2NH3 (complex 8) reveals the formation of a linear Ag(NH3)2+ cation non bonded to the metal cyanoxime anion 1−. This is the first structure of a silver(I) diammine cation with an oxime-based anion. Similarly to 8, the crystal structure of Ag(14ane[N4]){NC-C(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2}·CH3CN with tetraaza-meso(macrocyclic) ligand (complex 9) contains a metal center not bound to the cyanoxime ligand as well. Instead, the agostic interaction at 2.591 Å between the methylene group of the macrocyclic ligand and silver(I) center was found in 9. This is the shortest CH2–Ag distance between an aliphatic group and metal observed so far in non-organometallic silver complexes with neutral ligands. We also documented a remarkable visible-light insensitivity of complex 6. Room temperature solid state photoluminescence of this compound was examined in details, including studies of its emission in the presence of several gases of industrial importance: H2, CO, NO, NH3, SO2, acetylene C2H2 and ethylene C2H4. A significant sensitization of 6 to visible light after the exposure was observed, which is a useful property that may be utilized in development of battery-less colorimetric sensors for these gases.
O)-C(O)NH2}·CH3CN with tetraaza-meso(macrocyclic) ligand (complex 9) contains a metal center not bound to the cyanoxime ligand as well. Instead, the agostic interaction at 2.591 Å between the methylene group of the macrocyclic ligand and silver(I) center was found in 9. This is the shortest CH2–Ag distance between an aliphatic group and metal observed so far in non-organometallic silver complexes with neutral ligands. We also documented a remarkable visible-light insensitivity of complex 6. Room temperature solid state photoluminescence of this compound was examined in details, including studies of its emission in the presence of several gases of industrial importance: H2, CO, NO, NH3, SO2, acetylene C2H2 and ethylene C2H4. A significant sensitization of 6 to visible light after the exposure was observed, which is a useful property that may be utilized in development of battery-less colorimetric sensors for these gases.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OH)-C(O)NH2 (1), its
OH)-C(O)NH2 (1), its ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2} (complex 6) leads to a unique layered 2D coordination
O)-C(O)NH2} (complex 6) leads to a unique layered 2D coordination ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2}·2Pic (Pic =
O)-C(O)NH2}·2Pic (Pic = ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2}·2NH3 (complex 8) reveals the formation of a linear Ag(NH3)2+ cation non bonded to the metal
O)-C(O)NH2}·2NH3 (complex 8) reveals the formation of a linear Ag(NH3)2+ cation non bonded to the metal ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)-C(O)NH2}·CH3CN with
O)-C(O)NH2}·CH3CN with 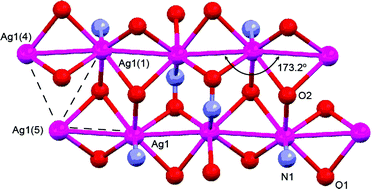

 Please wait while we load your content...
Please wait while we load your content...