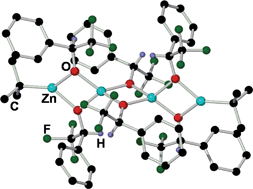
A systematic study of the stoichiometric alkylation reactions of 2,2,2-trifluoroacetophenone 1 with [ZnR2(TMEDA)] (R= Me, Et, tBu, CH2SiMe3; TMEDA= N,N,N′,N′-tetramethylethylenediamine) monitored by 1H and 19F NMR spectroscopy is presented. For R = Me, Et the alkylation products alkyl(alkoxides) [(TMEDA)Zn(R){OC(CF3)(R)Ph}] (R = Me, 2: Et, 3) are obtained as the single products of the reaction. When the steric bulk of the dialkylzinc reagent is increased the alkylation reaction is inhibited. Thus, for R = tBu, the reduction product [(TMEDA)Zn(tBu){OC(CF3)(H)Ph}] is obtained as a result of β-hydride elimination from one of the tBu groups of the organometallic reagent. 1H NMR spectroscopic monitoring of the reaction allowed the detection of isobutene as a side product of this reduction process. For the highly sterically demanding group R = CH2SiMe3 which lacks hydrogen atoms at the β position, no reaction is observed even under refluxing conditions. Two important intermediates from these reactions have been structurally elucidated: [(TMEDA)Zn(Me){OC(CF3)(Me)Ph}] (2) which could be involved in the previously reported alkylation reaction of trifluoromethyl ketones by ZnR2 catalysed by TMEDA and unprecedented tetranuclear [(tBu)2Zn4{OC(CF3)(H)Ph}6] (5) resulting from the reduction of 1 when reacted with tBu2Zn, which displays a rare Zn⋯Zn⋯Zn⋯Zn linear chain arrangement for a zinc alkyl(alkoxide).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...