Treatment of trimethylsilylethynylbenzene derivatives with HGaCl2 afforded products, [C6H6−x{C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(SiMe3)GaCl2}x], in which by a very fast cis/trans-rearrangement the Ga and H atoms occupied opposite sides of the resulting C
C(SiMe3)GaCl2}x], in which by a very fast cis/trans-rearrangement the Ga and H atoms occupied opposite sides of the resulting C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. The stability of the cis-forms considerably increased upon application of 1,3-dibromo- and pentafluorophenylalkyne derivatives. Two pairs of cis/trans-isomers could be characterized by crystal structure determinations and allow the direct comparison of structural parameters. For the first time an equilibrium was detected between cis- and trans-forms in solution. Treatment of 1,4-di(tert-butylalkynyl)benzene with HAlR2 (R = CMe3, CH2CMe3) afforded cyclophane-type molecules by the release of AlR3. Only the neopentyl derivative could be isolated and characterized by crystal structure determination. In contrast, the dibromo compound, 1,4-Br2-2,5-(Me3CC
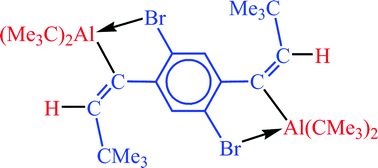
C double bonds. The stability of the cis-forms considerably increased upon application of 1,3-dibromo- and pentafluorophenylalkyne derivatives. Two pairs of cis/trans-isomers could be characterized by crystal structure determinations and allow the direct comparison of structural parameters. For the first time an equilibrium was detected between cis- and trans-forms in solution. Treatment of 1,4-di(tert-butylalkynyl)benzene with HAlR2 (R = CMe3, CH2CMe3) afforded cyclophane-type molecules by the release of AlR3. Only the neopentyl derivative could be isolated and characterized by crystal structure determination. In contrast, the dibromo compound, 1,4-Br2-2,5-(Me3CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H2, yielded the simple addition product, C6H2Br2{C(AlR2)
C)2C6H2, yielded the simple addition product, C6H2Br2{C(AlR2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)CMe3}2 (R = CMe3). Condensation was hindered in this case by intramolecular Al-Br interactions. Surprisingly, the simple addition product was also isolated from the reaction of 1,4-(Me3CC
C(H)CMe3}2 (R = CMe3). Condensation was hindered in this case by intramolecular Al-Br interactions. Surprisingly, the simple addition product was also isolated from the reaction of 1,4-(Me3CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4 with the relatively small hydride HAlEt2. Solid-state NMR spectra of the product revealed strong intermolecular Al–C interactions involving the negatively charged terminal vinylic carbon atoms, to give one-dimensional coordination polymers.
C)2C6H4 with the relatively small hydride HAlEt2. Solid-state NMR spectra of the product revealed strong intermolecular Al–C interactions involving the negatively charged terminal vinylic carbon atoms, to give one-dimensional coordination polymers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(SiMe3)GaCl2}x], in which by a very fast cis/trans-rearrangement the Ga and
C(SiMe3)GaCl2}x], in which by a very fast cis/trans-rearrangement the Ga and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. The stability of the cis-forms considerably increased upon application of
C double bonds. The stability of the cis-forms considerably increased upon application of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H2, yielded the simple addition product, C6H2Br2{C(AlR2)
C)2C6H2, yielded the simple addition product, C6H2Br2{C(AlR2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)CMe3}2 (R = CMe3). Condensation was hindered in this case by intramolecular
C(H)CMe3}2 (R = CMe3). Condensation was hindered in this case by intramolecular ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)2C6H4 with the relatively small
C)2C6H4 with the relatively small 

 Please wait while we load your content...
Please wait while we load your content...