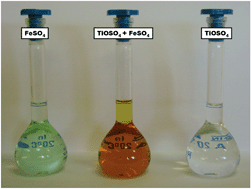
The titanyl equilibria in strongly acidic solutions related to the hydrometallurgical production of titania have been investigated in the presence of inorganic ions (ClO4−, NO3−, Cl−, SO42−, Fe2+ and Fe3+) using spectroscopic (UV–Vis and EPR) methods. UV–Vis experiments showed no interaction or ion pairing effect in the acidic titanyl nitrate and titanyl perchlorate samples indicating that they would be suitable background electrolytes for further thermodynamic measurements. The stoichiometry of the titanyl chloride complex was determined as TiOCl20 and its stability constant was calculated (log β2 = 1.12 ± 0.03). The interaction between titanyl and sulfate ions was quantified by determining the stoichiometry (1:1) and stability (log β1 = 1.64 ± 0.06) of the titanyl sulfate species. Weak sulfate complexes were also detected with the ferric (log β1 = 2.00 ± 0.10; log β2 = 2.49 ± 0.08) and ferrous (log β1 = 1.52 ± 0.06) ions under strongly acidic conditions. A double sulfato complex FeTiO(SO4)32− with relatively high stability (log β = 7.13 ± 0.07) was found in solutions containing titanyl, ferrous and sulfate ions in sulfuric acid medium. In addition, an EPR signal was assigned to the FeTiO(SO4)32− species. This double sulfato complex was found to be the major metal-containing species in the system similar to that used in the leaching process of ilmenite during the industrial production of titania.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...