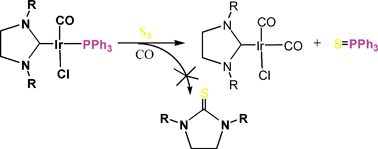
Iridium complexes [(CO)2Ir(NHC-R)Cl] (R = Et–, 3a; PhCH2–, 3b; CH3OCH2CH2–, 3c; o-CH3OC6H4CH2–, 3d; NHC: N-heterocyclic carbene) are prepared via the carbene transfer from [(NHC-R)W(CO)5] to [Ir(COD)Cl]2. By using substitution with 13CO, we are able to estimate the activation energy (ΔG‡) of the CO-exchange in 3a–d, which are in the range of 12–13 kcal mol−1, significantly higher than those for the phosphine analog [(CO)2Ir(PCy3)Cl]. Reactions of 3b and 3d with an equimolar amount of PPh3 result in the formation of the corresponding [(NHC-R)Ir(CO)(PPh3)Cl] with the phosphine and NHC in trans arrangement. In contrast, the analogous reaction of 3a or 3c with phosphine undergoes substitution followed by the anion metathesis to yield the corresponding di-substituted [(NHC-R)Ir(CO)(PPh3)2]BF4 (5) directly. Treatment of 3b or 3d with excess of PPh3 leads to the similar product of disubstitution 5b and 5d. The analysis for the IR data of carbonyliridium complexes provides the estimation of electron-donating power of NHCs versus phosphines. The NHC moiety on the iridium center cannot be replaced by phosphines, even 1,2-bis(diphenylphohino)ethane (dppe). All the carbene moieties on the iridium complexes are inert toward sulfur treatment, indicating a strong interaction between NHC and the iridium centers. Complexes 3a–c are active on the catalysis of the oxidative cyclization of 2-(o-aminophenyl)ethanol to yield the indole compound. The phosphine substituted complexes or analogs are less active.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...