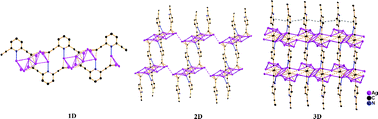
Two diethynylpyridines with different geometries were utilized to synthesize silver pyridyldiethynide complexes in order to investigate the relation between the structure of the coordination network and the geometry of the ligand as well as counter anion. Reactions of 2,5-diethynylpyridine with CF3CO2Ag and AgNO3 afforded [Ag2(2,5-C2PyC2)·4CF3CO2Ag] (1) and [Ag2(2,5-C2PyC2)·3AgNO3] (2), respectively, and a reaction of 2,6-diethynylpyridine with CF3CO2Ag gave [Ag2(2,6-C2PyC2)·3CF3CO2Ag] (3). X-Ray crystallographic studies revealed that in 1, 2,5-pyridyldiethynide groups as linkers connect silver chains to form a 2D organometallic network, and are further bridged by CF3CO2− anions to generate a 3D network, but in 2, 2,5-pyridyldiethynide groups link novel 2D silver layers to afford a 3D organometallic network. In 3, 2,6-pyridyldiethynide groups connect Ag10 units to generate an organometallic chain as bridging ligands, which are further linked by CF3CO2− ligands to form a 2D network. It is demonstrated that both the geometry of the ligand and coexisting anions are important factors that influence the structures of coordination networks based on silver pyridyldiethynide complexes.

 Please wait while we load your content...
Please wait while we load your content...