Carbon–hydrogenvs.carbon–halogen oxidative addition of chlorobenzene by a neutral iridium complex explored by DFT†
Abstract
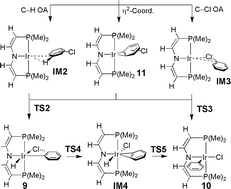
Density functional theory (DFT) is used to explore the competitive C–H and C–Cl oxidative additions (OA) of
- This article is part of the themed collection: The synergy between theory and experiment

 Please wait while we load your content...
Please wait while we load your content...