The role of the Pb2+ 6s lone pair in the structure of the double perovskite Pb2ScSbO6
Abstract
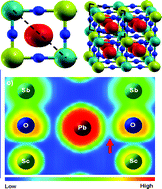
The new double perovskite Pb2ScSbO6 was synthesized by standard ceramic procedures; the Rietveld refinement of room temperature ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. It contains a completely ordered array of alternating ScO6 and SbO6 octahedra sharing corners; the PbO12 polyhedra present an off-center displacement of the lead atoms along the [111] direction, due to the electrostatic repulsion between the Pb2+ 6s lone pair and the Pb–O bonds of the cuboctahedron. Dielectric permittivity measurements show a peak near 343 K, with a Curie–Weiss response above this temperature, which suggests an antiferroelectric behavior. Finally we present a DFT study of the electronic structure of Pb2ScSbO6, showing a great difference between the electronic density within SbO6 and ScO6 octahedra.
m. It contains a completely ordered array of alternating ScO6 and SbO6 octahedra sharing corners; the PbO12 polyhedra present an off-center displacement of the lead atoms along the [111] direction, due to the electrostatic repulsion between the Pb2+ 6s lone pair and the Pb–O bonds of the cuboctahedron. Dielectric permittivity measurements show a peak near 343 K, with a Curie–Weiss response above this temperature, which suggests an antiferroelectric behavior. Finally we present a DFT study of the electronic structure of Pb2ScSbO6, showing a great difference between the electronic density within SbO6 and ScO6 octahedra.

 Please wait while we load your content...
Please wait while we load your content...