Formation of carbyne complexes in reactions of laser-ablated Os atoms with halomethanes: characterization by C–H(X) and Os–H(X) stretching absorptions and computed structures†
Abstract
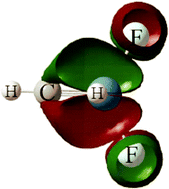
Reactions of laser-ablated Os atoms with
- This article is part of the themed collection: The synergy between theory and experiment

 Please wait while we load your content...
Please wait while we load your content...