Bonding properties and bond activation of ylides: recent findings and outlook
Abstract
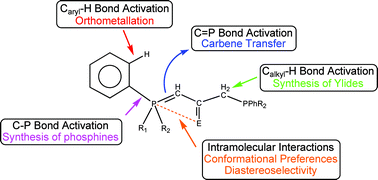
The interaction of phosphorus and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds as the source of these conformational preferences. The third topic is related to the amazing reactivity of
C hydrogen bonds as the source of these conformational preferences. The third topic is related to the amazing reactivity of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, P–C or C–H bond activation reactions.
C, P–C or C–H bond activation reactions.

 Please wait while we load your content...
Please wait while we load your content...