The copper(II)-mediated integration between various 3-iminoisoindolin-1-ones and neat organonitriles (which play a dual role of solvent and reactant), under heating for ca. 12 h, and without requiring any base, accomplishes the release of the solid (1,3,5-triazapentadienato)CuII complexes [Cu{HN=C(R)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C6R1R2R3R4CO)N}2] (1–11) bearing the incorporated iminoisoindolin-1-one fragment. These compounds were isolated in moderate to good yields (44–78%) and do not require further purification. The iminoisoindolinone–nitrile coupling is CuII-mediated and has a general character insofar as it can be efficiently applied for both unsubstituted (R1–R4 = H) and substituted iminoisoindolin-1-ones (R1, R3, R4 = H, R2 = Me; R1, R4 = H, R2, R3 = Cl; R1–R4 = F), and a wide range of nitriles (R = Me, Et, Prn, Pri, C6H11, CH2Ph). Complexes 1–11 were characterized by elemental analyses (C, H, N), FAB+-MS, IR spectroscopy, and additionally compounds 1, 2 and 4 by single-crystal X-ray diffraction.
C(C6R1R2R3R4CO)N}2] (1–11) bearing the incorporated iminoisoindolin-1-one fragment. These compounds were isolated in moderate to good yields (44–78%) and do not require further purification. The iminoisoindolinone–nitrile coupling is CuII-mediated and has a general character insofar as it can be efficiently applied for both unsubstituted (R1–R4 = H) and substituted iminoisoindolin-1-ones (R1, R3, R4 = H, R2 = Me; R1, R4 = H, R2, R3 = Cl; R1–R4 = F), and a wide range of nitriles (R = Me, Et, Prn, Pri, C6H11, CH2Ph). Complexes 1–11 were characterized by elemental analyses (C, H, N), FAB+-MS, IR spectroscopy, and additionally compounds 1, 2 and 4 by single-crystal X-ray diffraction.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C6R1R2R3R4CO)N}2] (1–11) bearing the incorporated iminoisoindolin-1-one fragment. These compounds were isolated in moderate to good yields (44–78%) and do not require further
C(C6R1R2R3R4CO)N}2] (1–11) bearing the incorporated iminoisoindolin-1-one fragment. These compounds were isolated in moderate to good yields (44–78%) and do not require further 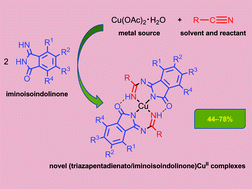

 Please wait while we load your content...
Please wait while we load your content...