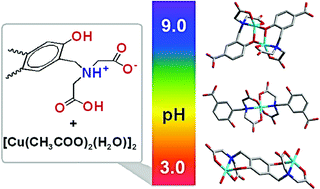
The reaction of Cu2+ acetate monohydrate with 2-[N,N′-bis(carboxymethyl)aminomethyl]-4-carboxyphenol (H4cacp), 2-[N,N-bis(carboxymethyl)aminomethyl]hydroquinone (H4cah) and the dinucleating 2,5-bis[N,N-bis(carboxymethyl)aminomethyl]hydroquinone (H6bicah) in water results in the formation of several Cu2+ species, which are in dynamic equilibrium in aqueous solution and their stability is pH dependent. A systematic crystallographic study of these species was pursued, resulting in the characterization of most of the species. Additional techniques were employed to characterize the molecules in the solid state (infrared spectroscopy) and in solution (UV-vis spectroscopy and electrochemistry). These measurements show that the Cu2+ ions are ligated mainly to the iminodiacetate at pH < 6, exhibiting only weak interactions with the phenol oxygen. At pH > 6, the phenol oxygen was deprotonated and dinuclear-bridged species, from the phenolate oxygen complexes exhibiting a Cu2+2O2 core, were isolated. The coordination environment around the copper ions varies between trigonal bipyramidal, tetragonal pyramidal and distorted octahedral geometries. The two unpaired electrons of the Cu2+ ions are found to be antiferromagnetically coupled. A survey of the magnetic and structural properties of the dinuclear phenoxide bridged Cu2+ complexes shows that the strength of the antiferromagnetic coupling is linearly dependent on the Cu–Ophenolate bond lengths, at bond distances below 1.98 Å. The effect of the Cu–O–Cu angles on the magnetic properties of the complexes is also discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...