Bis(phosphanylamino)benzeneligands: a zinc(ii) complex and an unusual nickel(i) complex with a Dewar-benzene-type Ni2P2N2 backbone†‡
Abstract
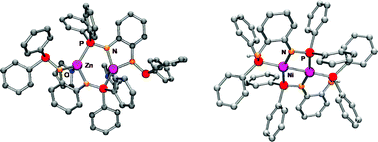
ZnPr2 reacts with 1,2-(NHPPh2)2C6H4 (1) to give the bis-amido complex [Zn(THF){1-N(PPh2)-2-N(μ-PPh2)C6H4-κ3N,N′,P}]2 (3), while monolithiated 1 (prepared in situ from 1 and LiBun) reacts with NiCl2 with formation of the unusual nickel(I) complex [Ni{1-NH(PPh2)-2-N(μ-PPh2)C6H4-κ2N,P}]2 (4), which has a Ni–Ni bond. Complexes 3 and 4 were structurally characterised. Furthermore, the structure of the sterically demanding bis-aminophosphine 1,2-(NHPMes2)2C6H4 (2, Mes = 2,4,6-Me3C6H2) is compared with that of the corresponding

 Please wait while we load your content...
Please wait while we load your content...