Sn-centred icosahedral Rh carbonyl clusters: synthesis and structural characterization and 13C–{103Rh} HMQC NMR studies†
Abstract
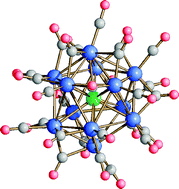
The reaction of [Rh7(CO)16]3− with SnCl2·2H2O in a 1 : 1 molar ratio under N2 results in the formation of the new heterometallic cluster, [Rh12Sn(CO)27]4−, in very high yield (ca. 86%). Further controlled additions of SnCl2·2H2O, or solutions of HCl, or [RhCl(COD)]2, give [Rh12(µ-Cl)2Sn(CO)23]4−. Similarly, addition of HBr to [Rh12Sn(CO)27]4− gives the related cluster [Rh12(µ-Br)2Sn(CO)23]4−. Notably, if the addition of SnCl2·2H2O to [Rh12Sn(CO)27]4− is carried out under a CO atmosphere, the reaction takes a different course and leads to the formation of the new cluster, [Rh12Sn(µ3-RhCl)(CO)27]4−. All the above clusters have been shown by single-crystal X-ray diffraction studies to have a metal framework based on an icosahedron, which is centred by the unique Sn atom. Their chemical reactivity and 13C–{103Rh} HMQC NMR spectroscopic characterization are also reported.

 Please wait while we load your content...
Please wait while we load your content...