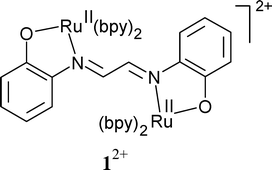
The rare bridging mode of 1,4-bis(2-phenolato)-1,4-diazabutadiene = glyoxalbis(2-hydroxyanil) (L2−) is adopted in {(µ-L2−)[RuII(bpy)2]2}2+ (12+), obtained as bis-perchlorate. Four well accessible redox forms of 1n (n = 4+, 3+, 2+, +) have been characterised by UV-VIS-NIR spectroelectrochemistry. The (3+) and (+) intermediates have also been investigated by EPR, both showing radical-type signals close to g = 2. This observation stands in stark contrast to EPR results previously obtained for the related {(µ-L)[Ru(acac)2]2}n, n = + and −, both of which exhibit metal-centred spin. In combination with the UV-VIS-NIR spectra these results suggest the preferential involvement of the multistep ligand redox system Ln− in the electron transfer processes. The relative stabilisation of RuII by π-accepting bpy is made responsible for the oxidation of the ligand L2− instead of the metal.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...