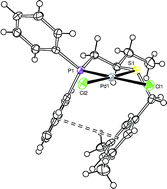
The synthesis of the benzyl phosphinothioether derivatives Ph2PCH2CH(Et)SR and their corresponding palladium complexes are reported, where R = CH2Ph (3), R = CH2-3,5-Me-C6H3 (4) and R = 1-CH2C10H7 (5). Crystallographic data obtained for the complexes Pd(3)Cl2 and Pd(4)Cl2 show intra- and inter-molecular π–π interactions between the aromatic rings on the P and S substituents, and NOE experiments for Pd(4)Cl2 show that these interactions persist in solution. The performance of the phosphinothioether palladium complexes in aryl–aryl cross-coupling reactions is compared with that of the corresponding complex of the parent phosphinothiolato ligand Ph2PCH2CH(Et)S− (1). High turnover numbers up to 2000000 are reported for the coupling of bromobenzene, using the palladium dimer [Pd(1)I]2 as the catalyst precursor. Kinetic studies show a linear dependence of the reaction on catalyst loading. The effect of other variables on the cross-coupling reaction, such as temperature, solvent and base, is also reported.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...