High-spin and low-spin iron(ii) complexes with facially-coordinated borohydride ligands†
Abstract
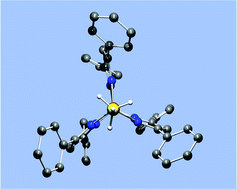
Rare examples of monometallic high-spin and low-spin L3Fe(H3BH) complexes have been characterized, where the two L3 ligands are [TpPh2] and [PhBP3] ([TpPh2] = [HB(3,5-Ph2pz)3]− and [PhBP3] = [PhB(CH2PPh2)3]−). The structures are reported wherein the borohydride ligand is facially coordinated to the iron center in each complex. Density functional methods have been employed to explain the bonding in these unusual iron(II) centers. Despite the differences in spin states, short Fe–B distances are observed in both complexes and there is significant theoretical evidence to support a substantial bonding interaction between the iron and boron nuclei. In light of this interaction, we suggest that these complexes can be described as (L3)Fe(η4-H3BH) complexes.

 Please wait while we load your content...
Please wait while we load your content...