The fixation of bis(2-pyridylimino)isoindolato (BPI) ligands to dendritic carbosilanes
Abstract
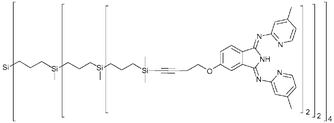
Bis(2-pyridylimino)isoindolato (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2CH2O]-10-MeBPI (8), [G-1]12-exo-4-[C
CCH2CH2O]-10-MeBPI (8), [G-1]12-exo-4-[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2CH2O]-10-MeBPI (9) and [G-2]16-exo-4-[C
CCH2CH2O]-10-MeBPI (9) and [G-2]16-exo-4-[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2CH2O]-10-MeBPI (10) were synthesized and fully characterized. The functional dendrimers were metallated by reaction with [(PhCN)2PdCl2] in
CCH2CH2O]-10-MeBPI (10) were synthesized and fully characterized. The functional dendrimers were metallated by reaction with [(PhCN)2PdCl2] in

 Please wait while we load your content...
Please wait while we load your content...