Structural studies of lanthanide ion complexes of pure gold, pure silver and mixed metal (gold–silver) dicyanides
Abstract
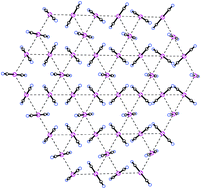
Crystal structures of four lanthanide complexes of La[Au(CN)2]3·3H2O, La[Ag(CN)2]3·3H2O, La[Ag0.83Au0.17(CN)2]3·3H2O, and La[Ag0.39Au0.61(CN)2]3·3H2O are reported. Studies reveal that all the structures reported are isostructural. All systems were found to be in the hexagonal crystal system, space group P63/mcm. The metal–metal distance for the pure gold system is 3.332 (1) Å versus 3.359(1) Å for the pure silver system. The mixed-metal systems have shown no distinct differences in the location of the metal atoms, with the La[Ag0.83Au0.17(CN)2]3·3H2O complex having a metal–metal Ag–Au separation of 3.346(1) Å, and 3.344(1) Å for the La[Ag0.39Au0.61(CN)2]3·3H2O complex. The crystal structures of the pure and mixed La complexes have been solved to provide evidence of Ag–Au heterometallic interactions and as a basis for understanding the interesting optical properties of the systems.

 Please wait while we load your content...
Please wait while we load your content...