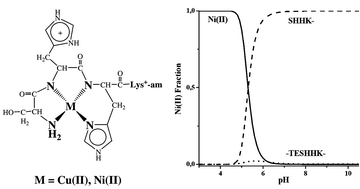
We studied the interactions of Ni(II) and Cu(II) ions with the synthetic tetrapeptides SHHK- and SAHK-, which were blocked by amidation making them more realistic models of the hydrolysis peptidic products of the hexapeptides models of H2A histone. A combination of potentiometric and spectroscopic techniques (UV/Vis, CD, NMR and EPR) suggested that at pH > 7 both tetrapeptides coordinated equatorially through the imidazole ring of His in position 3, the N-terminal amino group and the two amide nitrogens existing between these groups {NH2, 2N−, NIm} forming 4N square-planar complexes. While in the case of the CuH−1L complex with SHHK- a possible axial coordination of the imidazole ring of His in position 2 was suggested, in the case of the analogous NiH−1L complex a completely different interaction of the same ring with metal ions was observed. As expected these complexes have the same structures with the hydrolysis products produced from the Ni(II)- or Cu(II)-assisted hydrolysis of previously studied hexapeptide models of the C-terminal of histone H2A, due to their predominance at pH > 7.4. In addition, the competition plots presented herein showed that the synthetic tetrapeptides SHHK- and SAHK- have higher affinity towards Ni(II) and Cu(II) ions than the previously studied hexapeptides, suggesting that metal ions remain bound to the peptidic products during the hydrolysis cleavage. Thus, it can be concluded that the stability of Ni(II) or Cu(II) complexes with the synthetic tetrapeptides and consequently with the real hydrolysis peptidic products is the driving force of the hydrolysis reaction of H2A histone blocked hexapeptide models, presented in previous studies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...