Ferromagnetic exchange in a dinuclear copper(ii) complex mediated by a methanolate bridging ligand
Abstract
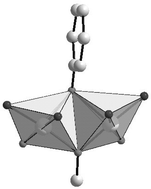
The copper(II) complex Cu2L(OMe)(H2O)3, 1·3H2O [H3L = 2-(2-hydroxyphenyl)-1,3-bis[4-(2-hydroxyphenyl)-3-azabut-3-enyl]-1,3-imidazolidine] was obtained and recrystallised in

 Please wait while we load your content...
Please wait while we load your content...