Iodide effects in transition metal catalyzed reactions
Abstract
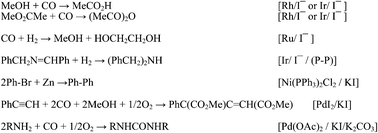
The unique properties of I− allow it to be involved in several different ways in reactions catalyzed by the late transition metals: in the oxidative addition, the migration, and the coupling/reductive elimination steps, as well as in substrate activation. Most steps are accelerated by I− (for example through an increased nucleophilicity of the metal center), but some are retarded, because a coordination site is blocked. The “soft” iodide ligand binds more strongly to soft metals (low oxidation state, electron rich, and polarizable) such as the later and heavier transition metals, than do the other halides, or N- and O-centered ligands. Hence in a catalytic cycle that includes the metal in a formally low oxidation state there will be less tendency for the metal to precipitate (and be removed from the cycle) in the presence of I− than most other ligands. Iodide is a good nucleophile and is also easily and reversibly oxidized to I2. In addition, I− can play key roles in purely organic reactions that occur as part of a catalytic cycle. Thus to understand the function of iodide requires careful analysis, since two or sometimes more effects occur in different steps of one single cycle. Each of these topics is illustrated with examples of the influence of iodide from homogeneous catalytic reactions in the literature: methanol carbonylation to acetic acid and related reactions; CO hydrogenation; imine hydrogenation; and C–C and C–N coupling reactions. General features are summarised in the Conclusions.

 Please wait while we load your content...
Please wait while we load your content...