Density functional calculations have been used to examine the reaction of {CpRe(CO)2} with fluorobenzenes C6FnH6−n
(n
= 0–5). Two classes of product have been observed experimentally (using Cp or Cp*): (a) coordination of the arene in an η2 fashion and (b) C–H activation to form a hydrido–aryl complex. Increasing the number of fluorines on the arene ring was shown to favour C–H activation. The thermodynamic and kinetic (reaction path) aspects of these transformations have been examined with DFT (B3PW91) calculations. For a given arene, the rhenium moiety is shown to exhibit the following order of thermodynamic preference for coordination: HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH site > HC
CH site > HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF site > FC
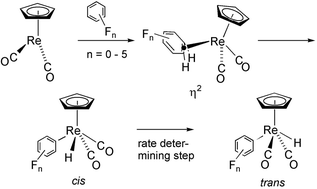
CF site > FC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF site. Binding energies to the different arenes do not follow a clear trend and span ca. 20 kJ mol−1. The Re–C bond energies in CpRe(CO)(H)(C6FnH5−n) span 55 kJ mol−1. Calculated structural parameters agree with the crystal structure of coordination of C6H6 and C6F6. Likewise the binding energy of C6H6 is in good agreement with experimental data. The calculated free energy difference between CpRe(CO)2(η2-C6FnH6−n) and CpRe(CO)2(H)(C6FnH5−n) shows that preference for the hydrido–aryl complex is determined principally by the bond dissociation energy of the C–H bond of the free arene. The binding energy to the η2-arene appears to be only a secondary factor. Three families of complexes are apparent. If there is no F on the carbon ortho to the Re–C bond that is formed, the η2-arene complex is energetically preferred. If there is one F at the ortho position, the energies of the products are similar. In the case of two ortho F substituents, the product of oxidative addition is significantly favoured. In agreement with the calculations, experimental evidence shows that benzene only coordinates to Cp*Re(CO)2, 1,4-C6F2H4 gives a mixture of products and 1,3-C6F2H4 gives only the hydrido–aryl complex. The arene with the stronger C–H bond is the one which gives more oxidative addition product because the Re–C bond energy increases with F substitution (and in particular with ortho F) more than twice as fast as the C–H bond dissociation energy. The reaction path for the overall transformation has been determined. The σ C–H complex is identified as an intermediate on the pathway for the oxidative addition. The initial product of oxidative addition is the cis hydrido–aryl isomer which subsequently isomerizes to the trans isomer. The rate determining step has been found to be the cis–trans isomerisation process and not the oxidation addition step. The cis–trans isomerisation proceeds via an unconventional concerted motion of H and the two COs. The variation of the Re–C bond energy is the dominant factor in determining the changes in the energy barrier between the different fluoroarenes, resulting in strong correlation between the thermodynamics and kinetics of reaction. The activation barriers are therefore also grouped in three families (0 F ortho, 1 F ortho, 2 F ortho).
CF site. Binding energies to the different arenes do not follow a clear trend and span ca. 20 kJ mol−1. The Re–C bond energies in CpRe(CO)(H)(C6FnH5−n) span 55 kJ mol−1. Calculated structural parameters agree with the crystal structure of coordination of C6H6 and C6F6. Likewise the binding energy of C6H6 is in good agreement with experimental data. The calculated free energy difference between CpRe(CO)2(η2-C6FnH6−n) and CpRe(CO)2(H)(C6FnH5−n) shows that preference for the hydrido–aryl complex is determined principally by the bond dissociation energy of the C–H bond of the free arene. The binding energy to the η2-arene appears to be only a secondary factor. Three families of complexes are apparent. If there is no F on the carbon ortho to the Re–C bond that is formed, the η2-arene complex is energetically preferred. If there is one F at the ortho position, the energies of the products are similar. In the case of two ortho F substituents, the product of oxidative addition is significantly favoured. In agreement with the calculations, experimental evidence shows that benzene only coordinates to Cp*Re(CO)2, 1,4-C6F2H4 gives a mixture of products and 1,3-C6F2H4 gives only the hydrido–aryl complex. The arene with the stronger C–H bond is the one which gives more oxidative addition product because the Re–C bond energy increases with F substitution (and in particular with ortho F) more than twice as fast as the C–H bond dissociation energy. The reaction path for the overall transformation has been determined. The σ C–H complex is identified as an intermediate on the pathway for the oxidative addition. The initial product of oxidative addition is the cis hydrido–aryl isomer which subsequently isomerizes to the trans isomer. The rate determining step has been found to be the cis–trans isomerisation process and not the oxidation addition step. The cis–trans isomerisation proceeds via an unconventional concerted motion of H and the two COs. The variation of the Re–C bond energy is the dominant factor in determining the changes in the energy barrier between the different fluoroarenes, resulting in strong correlation between the thermodynamics and kinetics of reaction. The activation barriers are therefore also grouped in three families (0 F ortho, 1 F ortho, 2 F ortho).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH site > HC
CH site > HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF site > FC
CF site > FC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF site. Binding energies to the different
CF site. Binding energies to the different 

 Please wait while we load your content...
Please wait while we load your content...