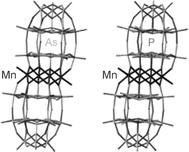
A tetranuclear manganous Wells–Dawson sandwich-type polyoxometalate has been synthesized by the reaction of α-Na12(As2W15O56) with an aqueous solution of MnCl2·4H2O. The structure of this complex, αββα-Na16(MnIIOH2)2MnII2(As2W15O56)2·55H2O (Na1), was determined by single-crystal X-ray crystallography (a
= 14.5230(12)
Å, b
= 14.7104(13)
Å, c
= 19.8927(17)
Å, α
= 84.326(2)°, β
= 81.709(2)°, γ
= 65.584(2)°, triclinic, R1
= 6.2%, based on 26721 independent reflections) and is similar to the phosphorus analogue, αββα-Na16(MnIIOH2)2MnII2(P2W15O56)2
(Na2). Magnetization studies confirm that both Na1 and Na2 show antiferromagnetic coupling of the four Mn(II) centers. Despite the structural similarities, electrochemical studies reveal that the presence of arsenic shifts the Mn waves to more positive potentials. Catalytic studies confirm that 1 is a significantly better catalyst than 2 for the H2O2-based epoxidation of cis-cyclooctene, cyclohexene, and 1-hexene.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...