The reaction of racemic 5,5′,6,6′- tetramethyl-3,3′-di-tert-butyl-1,1′-biphenyl-2,2′-diol (biphenolate-H2) with 4 mol equiv. of nBuLi yields [(μ3,μ3-biphenolate)2Li4(nBuLi)4]
(1) in high yield. 1 further reacts with 4 mol equiv. of 2,4-dimethyl-3-pentanol in the presence of tetrahydrofuran (THF) or cyclohexene oxide (CyHO) to give the lithium aggregate [(μ,μ-biphenolate)Li2(μ3-OCH(iPr)2)2Li2(L)2]
(2-THF, L = THF; 2-CyHO, L = CyHO). Treatment of biphenolate-H2 with 3 mol equiv. of ZnEt2, followed by addition of 2 mol equiv. of 2,4-dimethyl-3-pentanol provides the zinc complex [(μ,μ-biphenolate)Zn(μ-OCH(iPr)2)2Zn2Et2]
(3). Aluminium alkoxide incorporating biphenolate ligand can also be obtained via a similar synthetic route. The compound [(μ-biphenolate)AlMe(μ-OCH(iPr)2)AlMe2]
(4) is prepared from the reaction of biphenolate-H2 with 2 mol equiv. of AlMe3 in the presence of 1 mol equiv. of 2,4-dimethyl-3-pentanol. The titanium (4+) binolate complex [(biphenolate)Ti2Cl6]
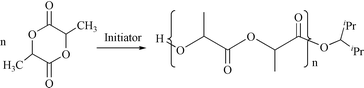
(5) is synthesized from the reaction of biphenolate-H2 and 2 mol equiv. of TiCl4. In addition, 2-THF, 3, and 4 have been examined for rac-lactide polymerization, and the comparative studies of polymerization are also presented.

 Please wait while we load your content...
Please wait while we load your content...