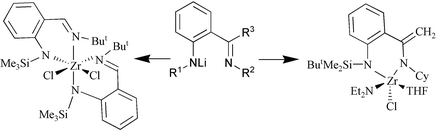
A range of trialkylsilylamido complexes of zirconium in which the silylamido functional group is attached to an o-(alkyliminomethyl)- or an o-(alkyliminoethyl)-substituted aromatic ring have been synthesised by salt elimination or aminolysis reactions and characterised by spectroscopic and diffraction methods. The monoanionic ligands (L), formally isoelectronic to the cyclopentadienyl ligand, can be introduced to zirconium by reaction of LLi with ZrCl4 under a variety of conditions giving rise to complexes of type LZrCl3 and L2ZrCl2, by reaction of LLi with Zr(NMe2)2Cl2(THF)2, and Zr(NEt2)2Cl2(THF)2 giving rise to LZrCl(NMe2)2Cl and LZrCl(NEt2)2Cl, respectively, or by reaction of LH with Zr(NMe2)4 giving rise to LZr(NMe2)3. LZr(NMe2)2Cl
and LZr(NEt2)2Cl can be transformed to LZrCl3 by reaction with Me3SiCl, while LZr(NMe2)3 on heating rearranges to dimeric imido complexes by elimination of amidosilanes. Aminolysis of Zr(NMe2)2Cl2(THF)2 with LH results in a formal insertion of the imino C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N into the Zr-amido bond. The combination of enolisable imine and a bulky silyl amide gave rise to a new mixed donor ligand system comprising of one silylamido and one eneamido group. Finally aminolysis of Ti(NMe2)Cl2 with LH resulted in the isolation of a C3 symmetric triamido titanium complex.
N into the Zr-amido bond. The combination of enolisable imine and a bulky silyl amide gave rise to a new mixed donor ligand system comprising of one silylamido and one eneamido group. Finally aminolysis of Ti(NMe2)Cl2 with LH resulted in the isolation of a C3 symmetric triamido titanium complex.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N into the Zr-amido bond. The combination of enolisable
N into the Zr-amido bond. The combination of enolisable 

 Please wait while we load your content...
Please wait while we load your content...