Palladium complexes containing bis(oxazolines): stoichiometric versus catalytic allylic alkylation
Abstract
The allylic palladium complexes (1L, for allyl = η3-C3H5; 2L, for allyl = η3-1,3-Ph2C3H3) with chiral 1,2-bis(oxazolinyl)benzene and 1,2-bis(oxazolinyl)ethane ligands L, (R,R)-A, (R,S )-A, (S,S
)-A, (S,S )-B, (R,R)-C, and (S,S
)-B, (R,R)-C, and (S,S )-D, were synthesized and fully characterized, both in solution and in the solid state. Five crystal structures of palladium allyl complexes are described, three of them containing the non-substituted allyl group, (R,S)-1A, (S,S)-1B, and (S,S)-1D, and two containing the 1,3-diphenylallyl group, (R,S)-2A and (S,S)-2B. A NMR study showed the existence of two isomers in solution for complexes containing 1,2-bis(oxazolinyl)benzene, endo and exo, with the diasteromeric excess of ca. 40% for type 1 complexes and ca. 75% for type 2 complexes. The catalytic behaviour of the palladium systems with the ligands described was tested for a model allylic alkylation reaction. The Pd/bis(oxazolinyl)benzene ((R,R)-A, (S,S
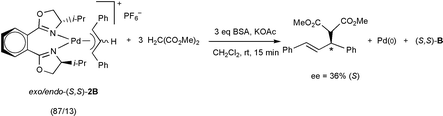
)-D, were synthesized and fully characterized, both in solution and in the solid state. Five crystal structures of palladium allyl complexes are described, three of them containing the non-substituted allyl group, (R,S)-1A, (S,S)-1B, and (S,S)-1D, and two containing the 1,3-diphenylallyl group, (R,S)-2A and (S,S)-2B. A NMR study showed the existence of two isomers in solution for complexes containing 1,2-bis(oxazolinyl)benzene, endo and exo, with the diasteromeric excess of ca. 40% for type 1 complexes and ca. 75% for type 2 complexes. The catalytic behaviour of the palladium systems with the ligands described was tested for a model allylic alkylation reaction. The Pd/bis(oxazolinyl)benzene ((R,R)-A, (S,S )-B) catalytic systems showed low activity, but good asymmetric inductions, affording enantiomeric excesses up to 86%. However, the Pd/bis(oxazolinyl)ethane systems exhibited lower activity and worse enantioselectivity than for the analogous catalysts containing the rigid phenyl backbone. Stoichiometric reactions, modelling the nucleophilic attack step of the catalytic cycle, from palladium(II) (2L) complexes, led to lower enantiomeric excesses than under catalytic conditions.
)-B) catalytic systems showed low activity, but good asymmetric inductions, affording enantiomeric excesses up to 86%. However, the Pd/bis(oxazolinyl)ethane systems exhibited lower activity and worse enantioselectivity than for the analogous catalysts containing the rigid phenyl backbone. Stoichiometric reactions, modelling the nucleophilic attack step of the catalytic cycle, from palladium(II) (2L) complexes, led to lower enantiomeric excesses than under catalytic conditions.

 Please wait while we load your content...
Please wait while we load your content...