Gold(i)-catalyzed cycloisomerization of ortho-(alkynyl) styrenes: DFT analysis of the crucial role of SbF6− in the elimination of protons†
Abstract
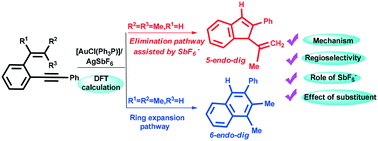
This study reports the first theoretical evidence for the crucial role played by SbF6− in the determination of the regioselectivity in the gold-catalyzed cycloisomerization of ortho-(phenylethynyl) styrenes. According to our calculations, irrespective of using α-substituted or β,β-disubstituted o-(phenylethynyl) styrene, a novel and unusual reaction pathway for elimination of protons involving a four-center-three-electron intermediate, instead of the generation of HSbF6, is observed. On account of the electronic effect, the ring expansion pathway is more favorable than the elimination pathway for α-substituted o-(phenylethynyl) styrene. By using β,β-disubstituted o-(phenylethynyl) styrene, an elimination pathway involving the four-center-three-electron intermediate affords the indenyl derivative. Based on our calculation results and experimental facts, the regioselectivity for the title reaction is mainly controlled by electronic effects rather than steric effects. The theoretical results not only well rationalize the experimental observations but also provide insights into the details of the reaction.


 Please wait while we load your content...
Please wait while we load your content...