The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2†
Abstract
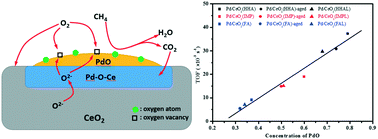
Three Pd/CeO2 catalysts are synthesized by reduction-deposition and an impregnation method (IMP) to clarify how the chemical state of Pd influences the catalytic performance for CH4 combustion. The results show that Pd/CeO2 (HHA), using hydrazine hydrate as a reductant, shows much higher catalytic activity and stability for CH4 combustion than Pd/CeO2 (FA), using formaldehyde as a reductant, and Pd/CeO2 (IMP). The results of our comprehensive characterization reveal that there are two different chemical states of Pd species on Pd/CeO2: Pd2+ in the form of PdO particles and Pdδ+ (2 < δ ≤ 4) in the form of PdxCe1−xO2, and the latter is mainly induced by the Pd species entering the lattice of CeO2, which acts as a transfer channel of active oxygen species from the CeO2 lattice to active Pd species. The activity of Pd/CeO2 directly correlates to the surface concentrations of PdO and adsorbed oxygen. An increase in the amount of PdxCe1−xO2 would thicken the transition layer and decrease the replenishment rate of oxygen vacancies through diffusion of lattice oxygen. Meanwhile, the existence of more PdxCe1−xO2 could promote the capture of produced CO2 on the CeO2 around Pd species and decrease the catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...