Conversion and coking of olefins on SAPO-34†
Abstract
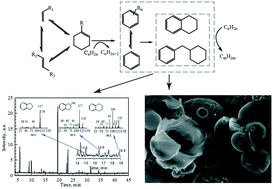
Conversion and coking of ethene, propene, 1-butene and their mixtures on SAPO-34 are investigated on a fixed bed reactor. Results indicate that 1-butene maintains a high conversion (ca. 97%), conversion of propene decreases from 64% to 28%, and ethene shows a very low conversion (ca. 14%). However, the conversion of ethene is improved by externally added or in situ formed propene and butene. This difference in olefin conversion is mainly related to the carbocationization tendency of olefins. The coking is also associated with the reactivity of olefins; moreover, the coking is related to the synergy effect between the diffusivity of olefins and the volumetric restriction of cages. Coke derived from the least diffusible 1-butene tends to have single and double ring structures. Coke derived from propene tends to form two or three fused ring structures. In addition, the most diffusible ethene favors the formation of three- or four-ring aromatics in SAPO-34 cages. Moreover, due to the alkylation across cage windows, well-bridged reticulate structure coke is formed in the SAPO-34 crystal. The effects of the olefins' further reaction on the MTO process are also discussed. It is supposed that the olefins' further reaction is one of the main sources of the coking. To design good performance MTO catalysts and to reveal the MTO mechanism, more attention should be paid to the effects of the olefins' further reactions.


 Please wait while we load your content...
Please wait while we load your content...