Ether formation through reductive coupling of ketones or aldehydes catalyzed by a mesoionic carbene iridium complex†
Abstract
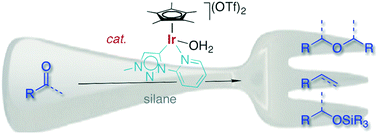
An iridium(III) Cp* complex containing a triazolylidene–pyridyl C,N-bidentate-coordinating ligand is a very powerful catalyst for the transformation of ketones and aldehydes into symmetrical ethers. This highly efficient reductive coupling proceeds immediately at room temperature and at a low catalyst loading (0.1 mol%) when Ph2SiH2 is used as an additive. Aromatic carbonyl substrates react faster than aliphatic ketones or aldehydes, and the substrate scope suggests some functional group tolerance. Likewise, the condensation of alcohols to symmetrical ethers is catalyzed by this triazolylidene iridium complex, though ether formation is an order of magnitude slower than when starting from the analogous ketone or aldehyde as a substrate, suggesting that alcohols are not potential intermediates in the reductive coupling process. Prolonged reactions or modification of the silane additive lead to ether cleavage and dehydration, thus affording the corresponding olefin. Mechanistic insights and in particular the different reactivities of alcohols and ketones have been exploited to develop a synthetic methodology for the iridium-catalyzed formation of unsymmetrical methyl ethers (R–OMe) in good yields.


 Please wait while we load your content...
Please wait while we load your content...