The role of a metallic copper interlayer during visible photocatalytic hydrogen generation over a Cu/Cu2O/Cu/TiO2 catalyst
Abstract
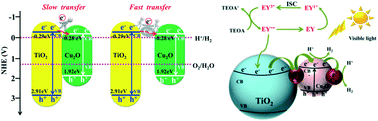
The role of metallic copper as a co-catalyst is still uncertain, which is an interesting problem in photocatalytic hydrogen evolution. There is debate over the role of Cu+, Cu2+ and metallic Cu during photocatalytic hydrogen generation. Herein, we identified that the metallic copper interlayer between Cu2O and TiO2 played a key role in the hydrogen evolution process. By fabricating the metallic Cu interlayer via in situ reduction of Cu2O/TiO2, efficient charge transfer took place over the Cu/Cu2O/Cu/TiO2 catalyst, while a retarded electron transfer was found over the Cu/TiO2 catalyst. This interlayer structure provides a bridge for photoelectron transfer from th`e conduction band of TiO2 to Cu2O, significantly prolonging the life-time of electron transfer. In addition, the ratio of metallic Cu and Cu2O could be easily adjusted by the loading amount of Cu2O on TiO2, and that the ratio affects the photocatalytic hydrogen evolution activity. A high transient photocurrent and long fluorescence lifetime (0.365 ns) were achieved over the Cu/Cu2O/Cu/TiO2 catalyst when the molar ratio of the interlayer metallic Cu to Cu2O was 0.99. Under the same reaction conditions, the photocatalytic hydrogen generation activity of the Cu/Cu2O/Cu/TiO2 catalyst was three times than that observed with the Cu/TiO2 catalyst with good stability.


 Please wait while we load your content...
Please wait while we load your content...