DFT and experimental analysis of aluminium chloride as a Lewis acid proton carrier catalyst for dimethyl carbonate carboxymethylation of alcohols†
Abstract
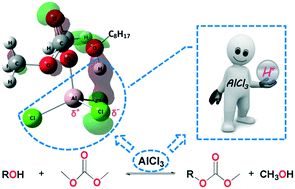
The Lewis acid catalysed mechanism of dimethyl carbonate (DMC) mediated carboxymethylation of alcohol was investigated experimentally and through computational chemistry methods including density functional theory (DFT). Experimental data showed that catalytic loading of AlCl3 enabled the quantitative carboxymethylation of octanol in less than 20 h, while in the absence of a catalyst only trace product was observed. The geometry of the identified transition states and related energy barriers indicate that the activation energies in AlCl3 catalysed pathways are significantly lower than those in catalyst-free pathways. Theoretical quantum chemistry methods were utilised to explore and analyse the complex of DMC with AlCl3. Natural bond orbital theory analysis and molecular orbital analysis demonstrated that the dipole present in Al–Cl covalent bonding plays a vital role in assisting the proton-transfer process. Most importantly, the reaction mechanism disclosed in this research can aid in the exploration of new Lewis acid catalysed processes in the field of dialkyl carbonate chemistry.


 Please wait while we load your content...
Please wait while we load your content...