Aliphatic Mn–PNP complexes for the CO2 hydrogenation reaction: a base free mechanism†
Abstract
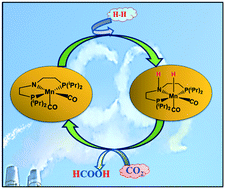
Using density functional theory (DFT) calculations, a series of aliphatic amido Mn–PNP-based complexes was studied for base free CO2 hydrogenation. The amido group present in the aliphatic PNP ligand favours a heterolytic H2 cleavage and proton transfer mechanism contrary to any active catalyst for CO2 hydrogenation, where base facilitates this process. Further, a set of σ-donor (PH3 and PMe3) and π-acceptor (CO and PF3) ligand-based Mn–PNP complexes was studied to understand the electronic and steric effects on the rate determining steps. Our reaction free-energy profile suggests that Mn1 is a promising base-free catalyst for CO2 hydrogenation with an overall free energy barrier of 23.3 kcal mol−1. We found that the proper combination of σ-donor and π-acceptor ligands may not be a necessary criteria to improve the overall activity of the catalyst although π-acceptor ligands favour heterolytic H2-cleavege and σ-donor ligands favour the hydride transfer mechanism.


 Please wait while we load your content...
Please wait while we load your content...