High activity and negative apparent activation energy in low-temperature CO oxidation – present on Au/Mg(OH)2, absent on Au/TiO2†
Abstract
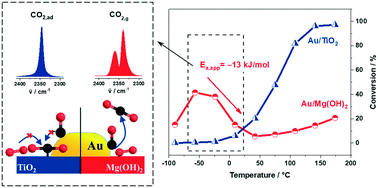
Aiming at a better understanding of the unusual low-temperature CO oxidation reaction behavior on Au/Mg(OH)2 catalysts, we investigated this reaction mainly by combined kinetic and in situ IR spectroscopy measurements over a wide range of temperatures, from −90 °C to 200 °C. Catalysts with a very narrow Au particle size distribution were prepared by colloidal deposition. Kinetic measurements, performed under differential, dry reaction conditions at different constant temperatures, enabled the separation of thermal and deactivation effects. They revealed that the distinct reaction behavior, with an exceptionally high activity at temperatures below 0 °C and decreasing CO oxidation rates in the range between −50 °C and 30 °C, equivalent to a negative apparent activation energy, does not result from either deactivation effects or H2O trace impurities, but is an intrinsic feature of the reaction. An unusual temperature dependence was also observed for the tendency for deactivation, with a pronounced maximum at −20 °C, which mainly results from an accumulation of surface carbonate species blocking active reaction sites or access of adsorbed reactants to them. Similar measurements on Au/TiO2 catalysts revealed that the high activity of Au/Mg(OH)2 in the low-temperature range compared to Au/TiO2 is first of all due to the weaker interactions of Mg(OH)2 with CO2 compared to TiO2. This leads to an increasing tendency of CO2 product molecules to adsorb on the latter catalyst at reaction temperatures below 0 °C and hence to rapid ‘self-poisoning’ with CO2 desorption as the rate-limiting step. For Au/Mg(OH)2, CO2 desorption is much faster, allowing much higher rates in the continuous CO oxidation. Based on temporal analysis of products (TAP) reactor measurements, the decay of the reaction rates in the range −50 °C to +50 °C is tentatively attributed to a decreasing steady-state coverage of weakly bound molecularly adsorbed O2 with increasing temperature, while stable adsorbed active surface oxygen is negligible over the entire range of reaction temperatures investigated. The implications of these and earlier findings for the mechanistic understanding of the low-temperature CO oxidation on Au/Mg(OH)2 and support effects therein are discussed.


 Please wait while we load your content...
Please wait while we load your content...