Brønsted acidic ionic liquid-catalyzed dehydrative formation of isosorbide from sorbitol: introduction of a continuous process†
Abstract
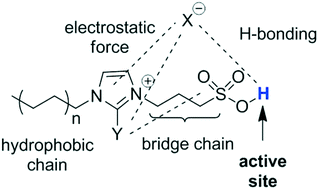
A highly efficient synthesis of isosorbide from sorbitol was developed using Brønsted acidic ionic liquids (BILs) as the catalyst for the first time. The structure–performance relationship was discussed extensively and a proper value of the Gutmann acceptor number (AN) rather than the inherent of acidity was found to be essential for an optimized yield of isosorbide. In addition, the excellent behavior of preferred BIL-4 in the consecutive recycling tests renders the construction of a continuous process probable. Systematic optimization demonstrated that a yield of 82% of isosorbide with a purity of 99.3% could be reached at balance.


 Please wait while we load your content...
Please wait while we load your content...