Electron-catalyzed radical perfluoroalkylation of organic sulfides: the serendipitous use of the TMEDA/I2 complex as a radical initiator†
Abstract
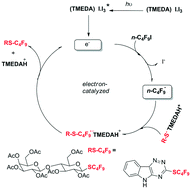
Radical initiation for the perfluoroalkylation reaction of sulfides has been performed using the complex [(TMEDA)I·I3] and visible light. This methodology bypasses the use of metal(organo)catalysts where the complex [(TMEDA)I·I3] acts as a good electron donor/reductant radical initiating agent. Biologically relevant sulfides are easily substituted with RF moieties employing a mild and environmentally benign radical strategy starting from readily available RFI.


 Please wait while we load your content...
Please wait while we load your content...