Comparative investigation of the deactivation behaviors over HZSM-5 and HSAPO-34 catalysts during low-temperature methanol conversion†
Abstract
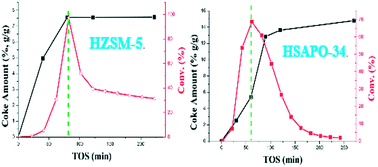
The deactivation mechanism for the methanol conversion reaction at low temperature was comparatively investigated over HZSM-5 and HSAPO-34 catalysts. Two obviously different deactivation phenomena were directly observed: two-staged deactivation behavior over the HZSM-5 catalyst and exponential-type deactivation behavior over the HSAPO-34 catalyst. Since the start of the deactivation, the amount of the retained species over the HZSM-5 catalyst kept unchanged while the amount over the HSAPO-34 catalyst obviously increased. Both types of deactivation behavior presented an intimate relationship with the accumulation of retained species and their changing reactivity. After detailed characterization and analysis, it was interestingly found that the deactivation of the HZSM-5 catalyst originated from the “overloading effect” of methylbenzenes (smaller than pentamethylbenzene) which are intrinsically active during the autocatalysis reaction stage, while the deactivation of the HSAPO-34 catalyst was caused by accumulation of inactive methyladamantanes, and it was further deduced that the deactivation proceeded from “external to internal” for the HSAPO-34 catalyst. Enhancement of the catalyst diffusivity could effectively extend the catalyst lifetime for the HZSM-5 catalyst, but seemed less effective for the HSAPO-34 catalyst.


 Please wait while we load your content...
Please wait while we load your content...