Abstract
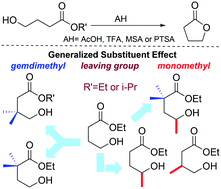
Intramolecular reactions affording cyclic compounds are key-transformations in organic chemistry. To better understand the factors that control the reaction rate of this important class of reactions, the acid catalysed ring closure of γ-hydroxyesters in a non protic solvent was investigated. First, a 1H NMR kinetic study of a set of monomethyl and/or gem-dimethyl substituted esters in CDCl3 was carried out. We evaluated the effect of the leaving group (ethyl vs. i-propyl ester) and the catalyst efficiency. We found that i) a monomethyl substitution produces a lowering of the energy barrier similar to that of a gem-dimethyl substitution (Thorpe–Ingold effect), ii) the ring closure of i-propyl esters is slower than that of ethyl esters, iii) strong acids are more efficient than weak acids according to the Brønsted relationship, and iv) the Thorpe–Ingold effect is not just an intrinsic feature of the linear precursor but depends on the catalyst as well. The reaction catalytic cycle was analyzed by DFT computations. The results of this analysis show that the generalized substituent effect (mono-, di- and trimethyl substitution together with the leaving group) can be explained in terms of ring strain energy, whereas the Brønsted plot is rationalized in terms of the atomic charge of the electrophile and the nucleophile/electrophile distance.


 Please wait while we load your content...
Please wait while we load your content...