Electrocatalytic behavior of freely-diffusing and immobilized synthetic flavins in aqueous media†
Abstract
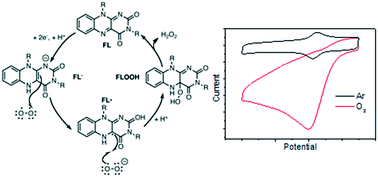
The electrocatalytic activity of three synthetic flavins in aqueous media toward the oxygen reduction reaction (ORR) was compared in this study. Two of the flavins (1 and 2) were in a freely-diffusing states while the third (flavin 3) was covalently-tethered to a glassy carbon (GC) electrode surface. The three synthetic flavin analogs were so designed such that the side groups of these molecules (on the C7, C8, N3, and N10 positions) were converted to impart greater stability (relative to natural flavins). The hydrogen on the N3 nitrogen was also replaced; in this way, no intramolecular proton-transfer could occur, leading to a simpler proton-coupled electroreduction mechanism. The experiments and kinetics analyses showed that an isoalloxazine structure for the flavin electrocatalyst (with a more positive E0 value) was better for electrocatalytic activity than the alloxazine counterpart. Importantly, covalent tethering of the molecule on the GC electrode surface had no deleterious influence on the ORR catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...