In situ electrochemical formation of NiSe/NiOx core/shell nano-electrocatalysts for superior oxygen evolution activity†
Abstract
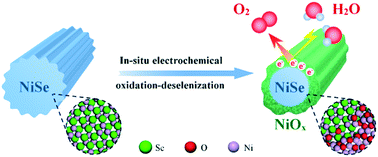
Despite the superior oxygen evolution electrocatalytic activity of metal-selenide nanostructures, especially when compared with their oxide counterparts, the origin behind their excellent activity remains unclear. Herein, we conduct a thorough and meticulous study on the NiSe oxygen evolution nanoelectrocatalyst—a representative metal selenide that was recently reported to be effective for the oxygen evolution reaction (OER). Our results unambiguously show that NiSe undergoes deselenization under electrocatalytic conditions, finally in situ transforming into a NiSe/NiOx core/shell nanostructure; the latter core/shell nanomaterial gives a superior activity towards OER. The NiSe/NiOx nanomaterial affords a current density of 10 mA cm−2 at an overpotential as low as ∼243 mV, outperforming most of the previously reported nickel (hydro)oxide OER electrocatalysts. These results further imply that the electrocatalytic activity of the so-called NiSe catalysts should originate from the in situ formed amorphous NiOx shell in nature, with the NiSe core having some auxiliary effects on the catalytic activity. Additionally, the NiSe/NiOx nanomaterial is also found to show a higher electrocatalytic activity than NiSe itself for the hydrogen evolution reaction (HER) in basic media.

 Please wait while we load your content...
Please wait while we load your content...