A novel Ce–Sb binary oxide catalyst for the selective catalytic reduction of NOx with NH3†
Abstract
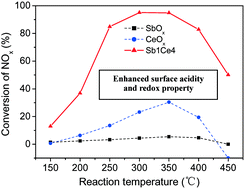
A series of Ce–Sb binary oxide catalysts prepared by the citric acid method have been investigated for the selective catalytic reduction (SCR) of NOx with NH3. It was found that the Ce–Sb oxide catalysts showed excellent NH3-SCR activity and a high tolerance to H2O and SO2 in a wide operation temperature window. N2-adsorption, X-ray diffraction (XRD), Raman spectroscopy, H2 temperature programmed reduction (H2-TPR), NH3 temperature programmed desorption (NH3-TPD), X-ray photoelectron spectroscopy (XPS) and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) were conducted to correlate the catalyst structure and surface properties to the catalytic performance of Ce–Sb binary oxide catalyst. The strong interaction between Sb and Ce species not only enhances the redox property of the catalyst but also increases the surface acidity, thus promoting the adsorption and activation of NH3 species, which is favorable for high NH3-SCR performance.

 Please wait while we load your content...
Please wait while we load your content...