The influence of the substituent on the phenol oxidation rate and reactive species in cubic MnO2 catalytic ozonation†
Abstract
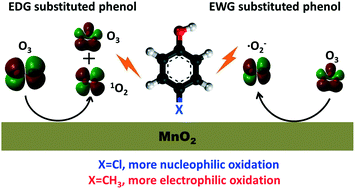
Shape-controlled MnO2, Mn2O3 and Mn3O4 were prepared and applied in catalytic ozonation of phenol (Ph), p-cresol (Ph-CH3) and p-chlorophenol (Ph-Cl). Their textural properties were characterized by XRD, SEM, TEM, nitrogen physical adsorption and CV. MnO2 was found to be more active than Mn2O3 and Mn3O4 for its higher electron transfer ability and higher amount of oxygen defects or surface hydroxyl groups. The degradation order was Ph-Cl > Ph-CH3 > Ph in the mixed solution, while the trend became Ph-CH3 > Ph-Cl > Ph in the single component solution, indicating that an electron donating group (EDG) and an electron withdrawing group (EWG) both benefit phenol degradation. The co-existing phenol had a different effect on the degradation of the other in the ozonation and catalytic ozonation of the mixed solution, but their influence on each other was universally opposite. Superoxide radical, singlet oxygen and molecular ozone were mainly responsible for phenol degradation in bulk solution. The contribution of ozone and singlet oxygen to the phenol degradation follows the order Ph-CH3 > Ph > Ph-Cl, indicating an electrophilic attack reaction, while the trend is opposite for superoxide radical oxidation, possibly due to a nucleophilic reaction pattern.

 Please wait while we load your content...
Please wait while we load your content...