Dual resistance to alkali metals and SO2: vanadium and cerium supported on sulfated zirconia as an efficient catalyst for NH3-SCR†
Abstract
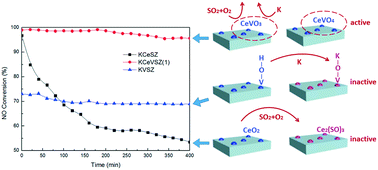
Vanadium and cerium supported on sulfated zirconia is an efficient SCR catalyst with dual resistance toward both potassium and SO2 poisoning. To the best of our knowledge, no examples of catalysts with a tolerance to both alkali metals and SO2 have been reported. Herein, the catalyst with a Ce : V molar ratio of 1 : 1 showed the highest activity (>97%) in the presence of potassium and SO2. The formation of thermally stable Ce2(SO4)3 disrupted the Ce(IV)/Ce(III) redox cycle, resulting in a significant deactivation of the CeSZ catalyst (93% to 63% in 400 min). Addition of a vanadium additive led to the transformation of CeO2 to CeVO4, thereby cutting off the reaction between CeO2 and SO2 and retaining the reactivity of the active sites. In addition, vanadium weakened the adsorption/oxidation of SO2 on the catalysts and thereby enhanced the tolerance toward SO2 by inhibiting the formation of ammonium bisulfate (NH4HSO4).

 Please wait while we load your content...
Please wait while we load your content...