Synthesis of high surface area CaxLa(1−x)Al(1−x)MnxO(3−δ) perovskite oxides for oxygen reduction electrocatalysis in alkaline media†
Abstract
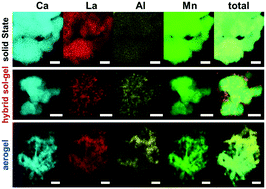
A series of perovskite oxide type catalysts with composition CaxLa1−xAl1−xMnxO1−δ were synthesized using solid state reaction, hybrid sol–gel, and aerogel synthesis techniques. The prepared catalyst materials were characterized with a suite of characterization techniques to determine morphology and composition. Electrochemical measurements for oxygen reduction reaction (ORR) activity in alkaline solution were performed using rotating disk electrode (RDE). ORR mass activity increased with increasing Brunauer–Emmett–Teller (BET) surface area, following the trend of solid state reaction < hybrid sol–gel < aerogel, when maintaining equal calcination time and temperature among all samples. Results also indicate a strong correlation between ORR specific activity and compositional homogeneity observed through transmission electron microscopy (TEM) with energy dispersive spectroscopy (EDS) mapping and X-ray photoelectron spectroscopy (XPS). Specifically, lower surface area materials produced by solid state reaction showed the highest compositional homogeneity and demonstrated highest specific activity. Performance tradeoffs are discussed relating surface area, compositional homogeneity at the oxide surface, and ORR activity.


 Please wait while we load your content...
Please wait while we load your content...