Selective direct hydroxylation of benzene to phenol with hydrogen peroxide by iron and vanadyl based homogeneous and heterogeneous catalysts†
Abstract
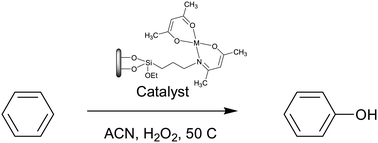
A series of first row transition metal complexes with Schiff base ligands or with readily available acetylacetonate ligands were screened as homogeneous catalysts in the challenging direct hydroxylation of benzene to phenol under mild conditions. Phenol was the main product of the oxidation of benzene in acetonitrile at 50 °C using hydrogen peroxide as the oxidant. The Fe(II/III) complexes with Schiff base ligands were the best homogeneous catalysts using only 1% mol based on the substrate with very high selectivities. All the Fe(II/III) complexes were more selective towards phenol than the VO(IV) complexes. The Fe(II/III) Schiff base complexes were more active than the readily available Fe(II/III) acetylacetonate complexes, whereas the opposite was observed for the VO(IV) complexes. The room temperature reaction gave a significantly lower phenol yield. Due to their easy anchoring, the readily available Fe(II), Fe(III) and VO(IV) acetylacetonate complexes were immobilized onto economical porous supports, hexagonal mesoporous silica and activated carbon, which were previously functionalized with amine groups. An Fe(III) Schiff base complex was also anchored onto these materials activated with a NaOH solution. The materials were characterized by elemental analysis, ICP-AES, FTIR spectroscopy, adsorption isotherm analysis at 77 K and TG analysis, showing that both porous materials were conveniently functionalized and that the metal complexes could be effectively anchored onto these materials. All the heterogeneous catalysts prepared were active and selective in the direct oxidation of benzene to phenol with higher yield and selectivity than the original metal salts or complex in homogeneous phase. For the immobilized Fe(II/III) acetylacetonate complexes, a significant improvement in the phenol yields was observed in comparison with the corresponding homogeneous phase catalysts, showing that the incorporation of nitrogen atoms on the metal coordination sphere improves the catalytic activity. The heterogeneous catalysts could also be recycled by simple filtration and re-used in at least three more consecutive cycles without significant loss of catalytic activity. Phenol yields comparable to or higher than the best reported in the literature were obtained.


 Please wait while we load your content...
Please wait while we load your content...